How to Determine if the Molecule Is Polar or Nonpolar
Draw the Lewis structure. Complete the following table.
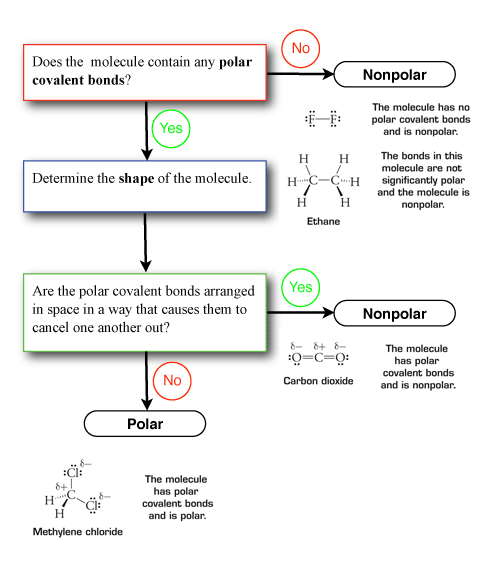
Unit 1 Elaboration Molecular Polarity
What is non polar compound.

. If there are no lone pairs on the central atom and if all the bonds to the central atom are the same the molecule is nonpolar. If the difference in electronegativity for the atoms in a bond is greater than 04 we consider the bond polar. Between two atoms that similarly share their electrons nonpolar bonds form.
The first three are symmetric shapes and the last two are asymmetric shapes. The bonds dont cancel each other out and are asymmetrical. A nonpolar molecule has no separation of electric charges or difference in electronegativity.
Next you have to examine the net partial charges. If the individual bond dipoles cancel the molecule is nonpolar. How to analyze the geometry of a molecule to decide whether a molecule that contains polar covalent bonds is polar or non-polar overall.
Note the number of electron regions around the central atom and of these which are bonding or lone pairs non-bonding pairs Step 2. Define polar and nonpolar molecule based on the activity. What are polar and non-polar molecules with examples.
Does it have a polar covalent bond. 7 How do you know a molecule is polar. As learned before non-polar molecules are perfectly symmetrical while polar molecules are not.
In order to determine whether a molecule is polar or nonpolar you must first examine its bonds. 8 Is hydrogen chloride polar or nonpolar. 10 Is hydrogen a polar.
If the result is between 0 and 1 then the bond is polarity. How do you determine if a molecule is polar or nonpolar given its structure. If a molecule contains no dipole moments across any bonds it will be nonpolar.
How do you determine polar and nonpolar without electronegativity. A molecule is polar if theres a significant difference in the electronegativity charges between elements. If not it is nonpolar.
How do you know if its polar or nonpolar. Bonding is usually referred to as a covalent bond which is called polar or non polar. Fats petrol oil gasoline are said to be non-polar molecules as they do not dissolve in water and nonpolar is insoluble in water.
If the difference in electronegativity is less than 04 the bond is essentially nonpolar If there are no polar bonds the molecule is nonpolar. Draw the Lewis structure. Glucose is one more example of a polar molecule based on.
These are problems using 3D molecules run in the application Jmol to help you visualize the molecule to determine if it is polar or non-polar. If the distribution looks even the molecule is nonpolar. Nonpolar molecule occur when electrons are shared equal between atoms or when polar bonds in a larger molecule cancel each other out.
As we can see polar molecules occur when is an electronegativity difference between the bonded atoms as this number 123 they are polar. When two bonded atoms exchange electrons in an unequal manner polar bonds form. This means that if the shape of the molecule given to you is.
14 What makes peroxide and water different. What about lone pairs. Figure out the geometry using VSEPR theory Visualize or draw the geometry.
The polarity of the bonds if they are polar also contributes to the polarity of the moleculeIf the shape is symmetric look to see whether all of the atoms attached to the central atom are the same. Polar molecules emerge because there is a difference in electronegativity between the bonded atoms. Otherwise it is polar.
Find the net dipole moment you dont have to actually do calculations if you can visualize it If the net dipole moment is zero it is non-polar. Learn to determine if a molecule is polar or nonpolar based on the polarity between bonds and the molecular geometry shapeWe start with the polarity betwe. Tell if the molecule is polar or nonpolar draw the Lewis dot structure for the molecule tell what shape the molecule is and state what the most significant intermolecular force affecting the molecule would be.
Keeping this in view why is it important to know if a molecule is polar or. If this is present then the molecule is polar. 9 What intermolecular forces are present in hydrogen peroxide.
When electrons are exchanged evenly between atoms in a diatomic molecule or when polar bonds in a larger. The first three are symmetric shapes and the last two are asymmetric shapes. Using numerical means find the difference between the electronegativity of the atoms in order to determine the polarity of a covalent bond.
A dipole exists when electrons are unevenly distributed from one side of the molecule to the other. If the central atom has at least one polar bond and if the groups bonded to the central atom are not all identical the molecule is probably polar. This means that if the shape of the molecule given to you is a bent or trigonal pyramid it is a polar molecule.
Since electrons are more attracted to oxygen than hydrogen they tend to congregate on that end of the molecule. Use this info to determine the 3D geometry of the molecule. If there is a net partial charge the molecule is polar.
A polar covalent bond is generally found in bonds of 7. 11 How do you determine polar and nonpolar. 12 What makes water a polar molecule.
Both polar and nonpolar molecule experience polarization on exposure to the electric field but the difference between a nonpolar and a polar molecule is that nonpolar molecules are induced with a dipole by current whereas polar molecules have permanent dipoles. 13 How does hydrogen peroxide react with water. For a molecule that has polar bonds the molecular geometry must be known in order to predict the overall polarity.
As learned before non-polar molecules are perfectly symmetrical while polar molecules are not. The lone pair or pairs of electrons on the central atom guarantee a nonuniform distribution of electrons.

Polar And Nonpolar Molecules How To Tell If A Molecule Is Polar Or Nonpolar Youtube
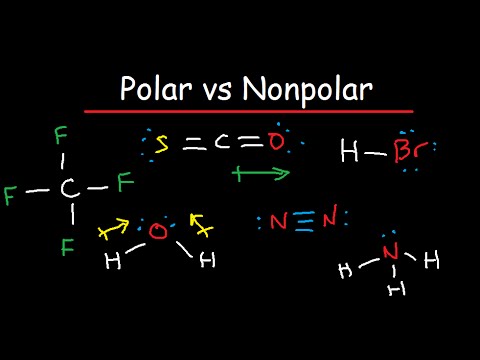
Polar And Nonpolar Molecules Is It Polar Or Nonpolar Youtube

4 3 Polarity Of Bonds And Molecules Chemistry Libretexts
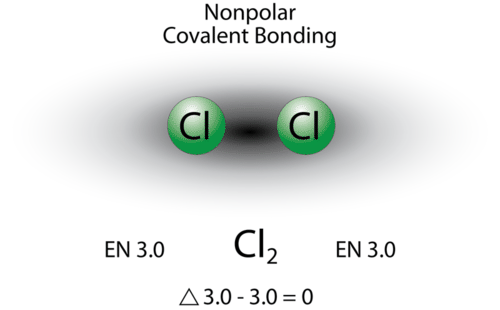
5 3 Polarity And Intermolecular Forces Chemistry Libretexts
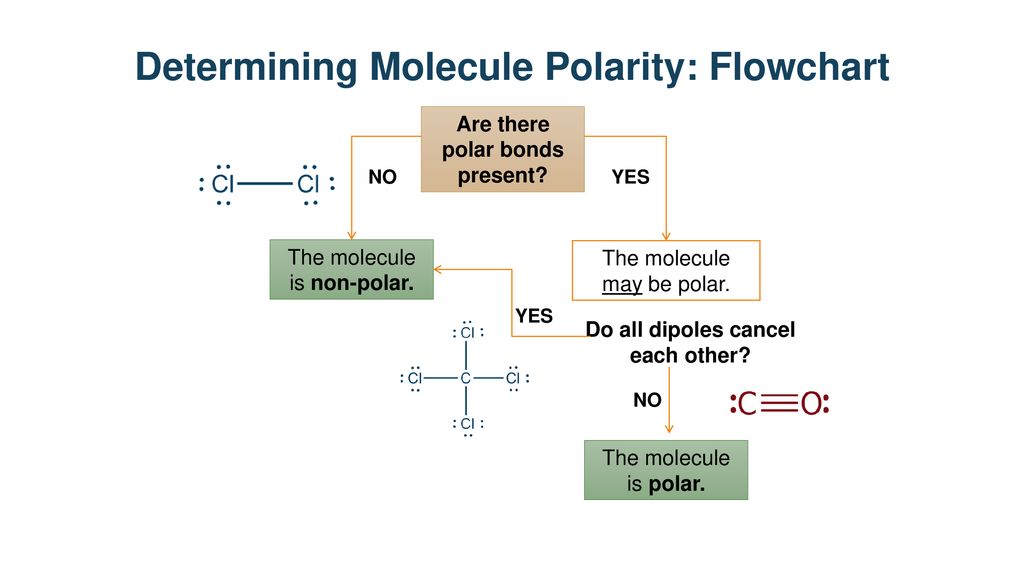
Polar Molecules Section Ppt Download
How To Determine The Type Of Molecule As To Polar Or Nonpolar Molecule That Will Form Between Each Pair Of Atoms Quora

Polar Molecules Chemistry For Non Majors
How Can Vsepr Theory Be Used To Find Out If A Molecule Is Polar Or Nonpolar Quora
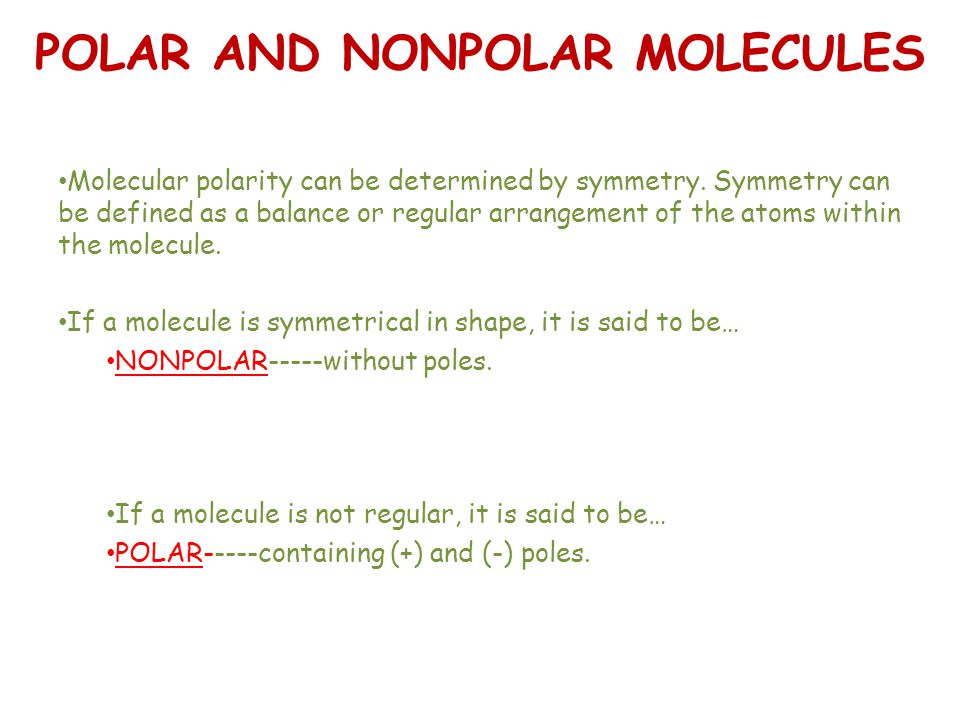
Polar And Nonpolar Molecules Molecular Polarity Can Be Determined By Symmetry Symmetry Can Be Defined As A Balance Or Regular Arrangement Of The Atoms Ppt Download
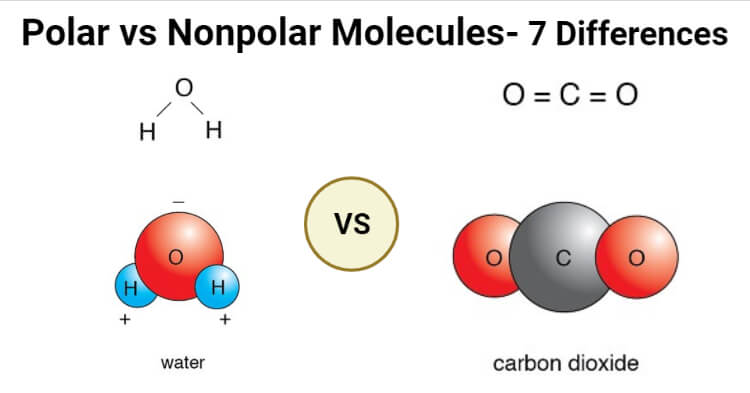
Polar Vs Nonpolar Molecules Definition 7 Key Differences Examples

Polar And Nonpolar Molecules Youtube

Unit Bonding Tier 5 Determine If A Molecule Is Polar Or Nonpolar Ppt Video Online Download

To Determine If A Molecule Is Polar Or Nonpolar How Do I Determine The Electronegativity Difference Homeworklib

Polarity Of Molecules Objectives 1 State The Two Factors That Determine Polarity Of A Molecule 2 Explain How The Structure Of A Molecule Helps Determine Ppt Download
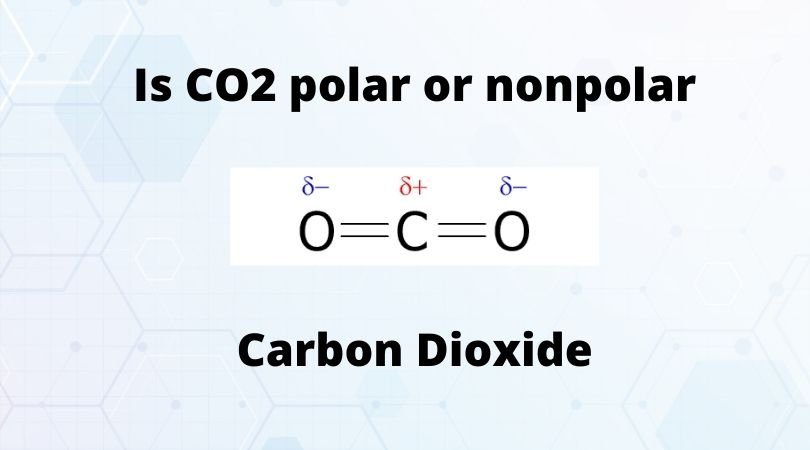
Is Co2 Polar Or Nonpolar Check Carbon Dioxide Polarity Geometry Of Molecules

How To Determine If A Molecule Is Polar Or Non Polar Check Now

Lesson Explainer Polar And Nonpolar Solvents Nagwa

How To Determine If Molecule Is Polar Or Nonpolar Practice Problems Rules Examples Summary Youtube

Comments
Post a Comment