Which Best Explains the Surface Tension of Water Brainly
Please mark this as the brainiest answer. This week we will see how the surface tension of water can be really pushed to the limit in a couple of simple experiments.
6 Why is the density of water important.

. 2 What are the two reasons why ice floats. We know that F ma substituting the value in the equation we get. 7 Why is density of ice less than water.
The lower the temperature the. 8 What if water has no surface tension. Its hydrogen bonding interactions.
Which best explains the high surface tension of water. As we know surface tension is given by the formula Surface tension FL. 4 Why does ice float on water and why is it important.
It is the concept pf surface tension. Cohesion is the attraction of water molecules with other water molecules Waters ___________________ explains why this substance helps to regulate body temperature as well as the Earths climate. One called Pennies in a cup and the other Drops of water on a penny Match with the search results.
1 Explain Why Ice Floats. 3 Why does ice float in water answer choices. MLT -2 L -1.
6 Why does water have a high surface tension Brainly. 02122020 0443 PM Average star voting. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds water has a higher surface tension 728 millinewtons mN per meter at 20 C than most other liquids.
Equating the fundamental quantities into the equation we get. It tends to aggregate in drops rather than. Mark it as brainliestplease.
Surface tension is an important factor in the phenomenon of capillarity. Surface tension in water might be good at performing tricks such as being able to float a paper clip on its surface but surface tension performs many more duties that are vitally important to the environment and people. 3 25057 reviews Summary.
10 How do you explain the fact that water has the highest surface tension but has the lowest viscosity. Hence the dimensional formula of surface tension is MT-2. Surface tension decreases with increasing temperatureTemperature has an effect on intermolecular forces.
I hope that this is the answer that has actually come to your great help. 8 When water freezes ice floats Why quizlet. Solving further we get.
9 What would happen if water has weak surface tension. Which property of water best explains the high surface tension. Hope this helps.
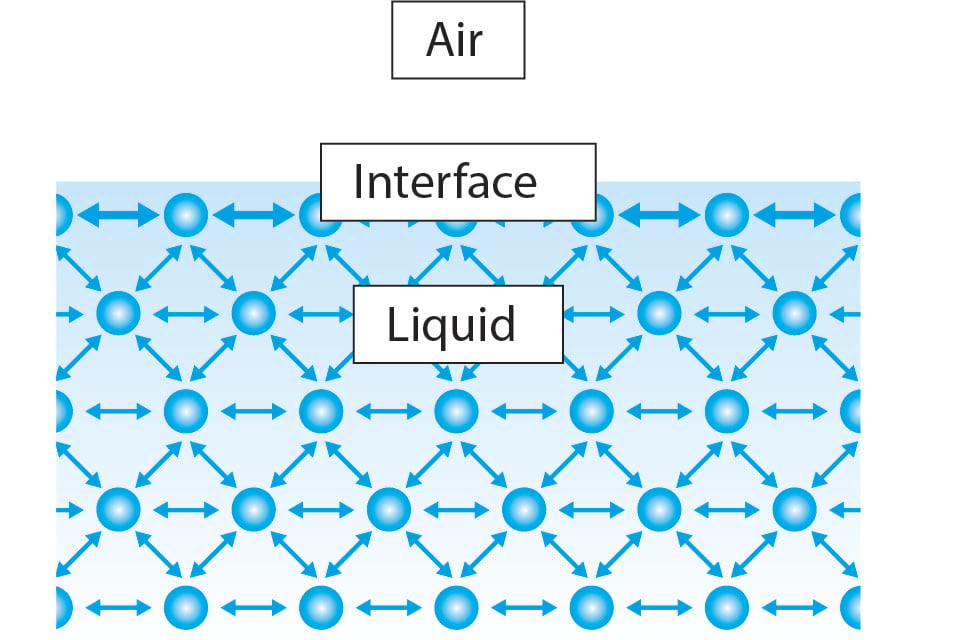
Cohesion is the one among the following choices given in the question that best explains the surface tension of water. Cohesion is a property of water that enables the high surface tension. Find out all about surface tension and water here.
The higher the temperature the greater the kinetic energies of the molecules and the greater the extent to which their intermolecular forces are overcome and so the more fluid less viscous the liquid. 7 Why does water have an increased surface tension compared to most other liquids. 5 Why does ice float on water by Brainly.
Cohesion is the one among the following choices given in the question that best explains the surface tension of water.

Why Are Earthquakes Common In The Pacific Ring Of Fire Science In Depth Reporting On Science And Technology Dw 15 02 2021

Materi Kalimat Pasif Bahasa Inggris Dengan Latihan Soal Nasional Katadata Co Id

Paper Clip Can Float On Water Due To High Surface Tension Of Water U S Geological Survey

A1d966cb 2b04 4e2e 87dd 25b875397ce9 Pdf

Why Are Earthquakes Common In The Pacific Ring Of Fire Science In Depth Reporting On Science And Technology Dw 15 02 2021

Why Are Earthquakes Common In The Pacific Ring Of Fire Science In Depth Reporting On Science And Technology Dw 15 02 2021

Science Curriculum Lifecycle Of A Frog Frog Life Frog Life Cycle Video

What Are The Functions Of The Italicized Words Dialog 3 Bantu Jawab Please Brainly Co Id

Surface Tension Of Water Why Is It So High

Why Are Earthquakes Common In The Pacific Ring Of Fire Science In Depth Reporting On Science And Technology Dw 15 02 2021








Comments
Post a Comment